Abstract
Introduction: First and second generation TKIs are established for the up-front treatment of chronic myeloid leukemia (CML). An increased bleeding risk in some patients on dasatinib is described but the underlying mechanism is not yet clear.
Aims: We collected blood samples from 15 healthy young adult volunteers and investigated platelet function and hemostasis screening testing after incubation with TKIs imatinib, nilotinib, dasatinib. and bosutinib in vitro .
Methods: Citrated whole blood (WB) was incubated for 30 min with TKI at different final concentrations (imatinib 1.5 µmol/l, nilotinib 4 µmol/l, dasatinib 0.2 and 0.4 µmol/l, bosutinib 0.4 µmol/l) reflecting clinically relevant blood TKI concentrations. Platelet function (aggregation and ATP release) was investigated by the Chronolog lumi-aggregometer type 560 (Chronolog Corp., Havertown, USA) using collagen (1 µg/ml), arachidonic acid (0.5 mmol/l), and thrombin (0.5 U/ml). Function of primary hemostasis was screened by the PFA-100® test (Siemens Dade Behring, Marburg, Germany) measuring the closure time (CT). Buffered citrated WB specimen without TKI served as controls.
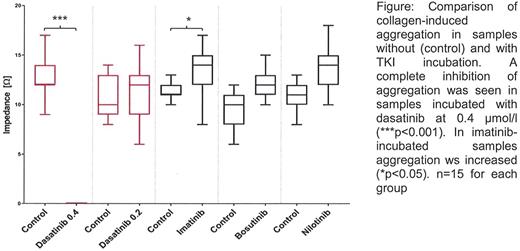
Results: Dasatinib at 0.4 µmol/l almost completely inhibited collagen-induced aggregation (see figure) and caused a marked CT prolongation with the collagen/epinephrine cartridge. Contrastingly, platelet activation by the other agonists (arachidonic acid, thrombin) and the CT with the collagen/ADP cartridge was not influenced. The concentration dependency of this effect was demonstrated using dasatinib at 0.2 µmol/l.
In imatinib-incubated samples the collagen-induced aggregation was significantly increased compared to controls while for the other parameters no significant differences were found. The PFA-100® CT was not influenced by imatinib. Comparable results were obtained for bosutinib and nilotinib.
Conclusions: Our data clearly demonstrate that dasatinib but not imatinib, bosutinib and nilotinib impair primary hemostasis by impairing the collagen-mediated platelet activation. The underlying precise molecular mechanism still awaits further elucidation. As CML is mostly diagnosed in elderly people, clinical relevance of these findings must be judged on in the context of underlying vascular endothelial diseases.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal